ABOUT

My webpage will soon move to https://www.miriammelislab.it/
The aim of our research is to understand neurobiological underpinnings (e.g., adaptations of both mesocorticolimbic dopamine pathway and endocannabinoid system) of susceptibility and resilience to neuropsychiatric disorders (e.g., substance use disorder, antisocial behavior, anxiety, depression).
Whether or not these are innate (i.e., temperaments, sex) or induced by early life adverse events (e.g. drug exposure, adverse social events) will be thoroughly investigated via an integrative approach including cellular physiology, neuroanatomy, molecular and behavioral analysis.
This is based upon my personal interest in neuropsychiatric disorders, particularly those mirroring affective imbalances within the brain. Affects are essential for well-being, and once primary physiological needs and safety are satisfied, interpersonal interaction and acceptance represent needs that have to be met, and drive motivation. In fact, they act as natural reinforcers, and are important for social and cognitive development.
The mesocorticolimbic dopamine system, key component of brain reward circuitry, processes value-related signals, and is considered an important circuit for affective functioning. Modulation of pathways determining the strength and direction of synaptic plasticity of such a system is, therefore, crucial in regulating experience-dependent behavioral changes, and viceversa. In particular, the individual response to early life adverse experience depends upon an organism’s innate biological make-up.
The findings will enable to develop new pharmacological strategies for the prevention and treatment of aberrant affective functioning, especially by investigating the mechanisms of resilience.
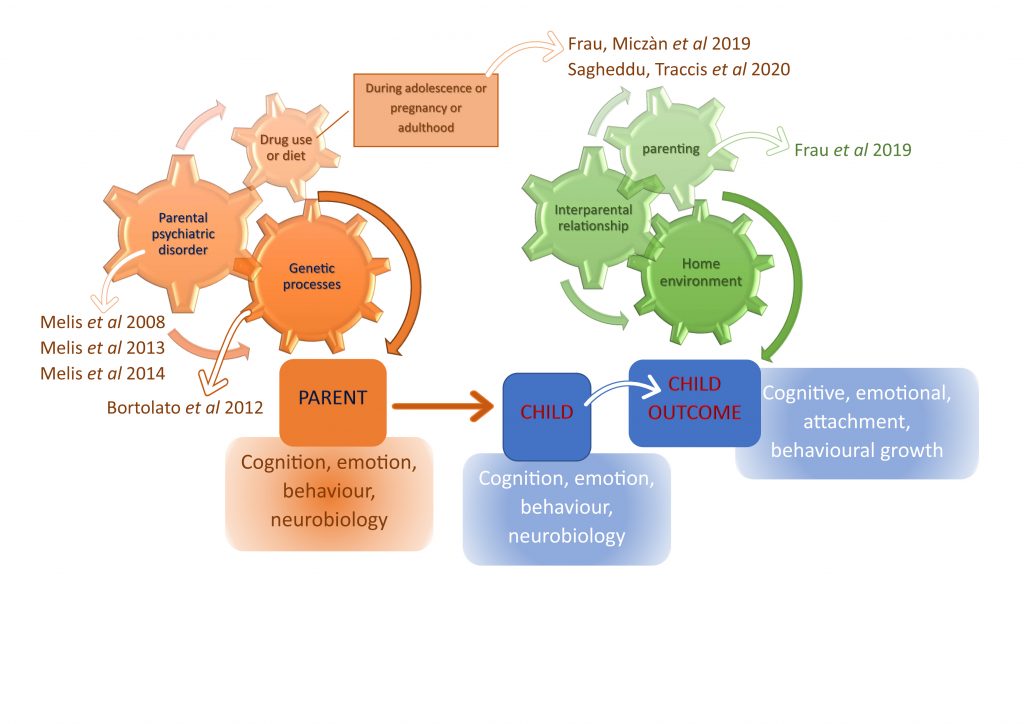
We began to address this issue by examining those associations between parental disorders and offspring outcomes from fetal development to adolescence (see selected publications below).
We aim to specifically assess the role of mediating variables, underlying the links between parent psychopathology and offspring outcome, and possible moderators, which may change the strength of any given association, and focus on factors that are potentially modifiable (see diagrams at the end of the page).
Sagheddu C, Traccis F, Serra V, Congiu M, Frau R, Cheer JF, Melis M* (2021) Mesolimbic dopamine dysregulation as a signature of information processing deficits imposed by prenatal THC exposure. Prog Neuropsychopharmacol Biol Psychiatry. doi: 10.1016/j.pnpbp.2020.110128.
Frau R, Miczán V, Traccis F, Aroni S, Pongor CI, Saba P, Serra V, Sagheddu C, Fanni S, Congiu M, Devoto P, Cheer JF, Katona I, Melis M* (2019) Prenatal THC exposure produces a hyperdopaminergic phenotype rescued by pregnenolone Nat Neurosci. 22: 1975–1985; doi: 10.1038/s41593-019-0512-2.
Frau R, Fanni S, Serra V, Simola N, Godar SC, Traccis F, Devoto P, Bortolato M, Melis M* (2019) Dysfunctional mesocortical dopamine circuit at pre-adolescence is associated to aggressive behavior in MAO-A hypomorphic mice exposed to early life stress. Neuropharmacol. doi: 10.1016/j.neuropharm.2019.01.032
Melis M*, Sagheddu C, De Felice M, Casti A, Madeddu C, Spiga S, Muntoni AL, Mackie K, Marsicano G, Colombo G, Castelli MP, Pistis M. (2014) Enhanced endocannabinoid-mediated modulation of rostromedial tegmental nucleus drive onto dopamine neurons in Sardinian alcohol-preferring rats. Journal of Neuroscience, 34(38):12716-24
Melis M*, De Felice M, Lecca S, Fattore L, Pistis M (2013): Sex-specific tonic 2-arachidonoylglycerol signaling at inhibitory inputs onto dopamine neurons of Lister Hooded rats. Frontiers in Integrative Neuroscience, 7(93):1-13.
Bortolato M, Godar S, Melis M, Soggiu A, Roncada P, Casu A, et al. (2012) NMDA receptors mediate the role of monoamine oxidase A in pathological aggression. Journal of Neuroscience, 32(25):8574-82.
*CA
 From letf: Dopamine (TH, green) neurons backfilled by including biocytin (red) in the recording pipette during whole cell patch clamp experiment. Current clamp traces of dopamine cell spontaneous activity (in red overlaid) and, on the right, DIC image of a putative dopamine neuron (*).
From letf: Dopamine (TH, green) neurons backfilled by including biocytin (red) in the recording pipette during whole cell patch clamp experiment. Current clamp traces of dopamine cell spontaneous activity (in red overlaid) and, on the right, DIC image of a putative dopamine neuron (*).
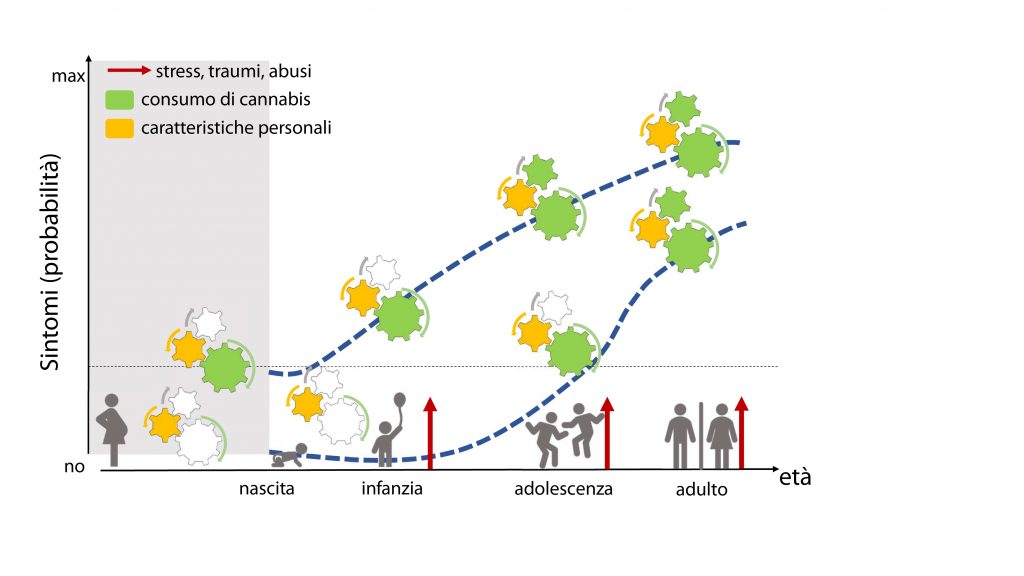
Diagram illustrating the risk factors leading to an at-risk endophenotype for neuropsychiatric disorders. We consider those associations between parental disorders and child outcome from fetal development to adolescence, but focus on the vulnerable period preceding adolescence. The interaction among biological make up of the individual, environment (e.g. one or both parental drug use, parental neglect, peer influence, etc) and age-related effects of indirect (i.e. pre/peri-natal exposure to drugs of abuse such as cannabis derivatives) and direct (early onset drug use) results in changes at cellular and synaptic level that contribute to the development of an at-risk endophenotype for neuropsychiatric disorders.
Benvenuti nel sito di Miriam Melis
- Ruolo:
- Professore Associato
- Area scientifico disciplinare:
- Scienze biologiche
- Settore scientifico disciplinare:
- BIO/14 FARMACOLOGIA
- Dipartimento di afferenza:
- Dipartimento di Scienze Biomediche
- Contatti:
- Email myriam@unica.it - Tel. 070/675-4322 -4340 fax: 070/675-4320
- Orario di ricevimento:
- Ricevo previo appuntamento via email